Next: Magnetisation Up: Magnetic Measurements Previous: Magnetic Measurements Contents
A magnet in a field has a potential energy, ![]() , relative to the parallel position given by
, relative to the parallel position given by
| (3.1) |
The origin of atomic magnetic moments is the incomplete cancellation of electronic magnetic moments. Electron spin and orbital motion both have magnetic moments associated with them but in most atoms the electronic moments are oriented so that they cancel giving no net atomic magnetic moment, leading to diamagnetism. If the cancellation of electronic moments is incomplete then the atom has a net magnetic moment. These ``magnetic atoms'' can display para-, ferro-, antiferro- or ferrimagnetic ordering depending upon the strength and type of magnetic interactions and external parameters such as temperature.
The magnetic moments of atoms, molecules or formula units are often quoted in terms of the Bohr magneton3.1, which is equal to the magnetic moment due to electron spin
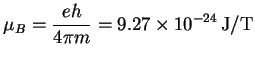 |
Dr John Bland, 15/03/2003